

Mechanism
of action
For adults with moderately to severely active ulcerative colitis (UC) or Crohn's disease (CD).
address inflammation where it occurs
ENTYVIO is a gut-selective biologic
It specifically binds to the α4β7 integrin and blocks its interaction with MAdCAM-1, which is mainly expressed on gut endothelial cells.1-7
For adults with moderately to severely active ulcerative colitis (UC) or Crohn’s disease (CD).
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
ENTYVIO is contraindicated in patients who have had a known serious or severe hypersensitivity reaction to ENTYVIO or any of its excipients.
Please listen for additional Important Safety Information during this video.
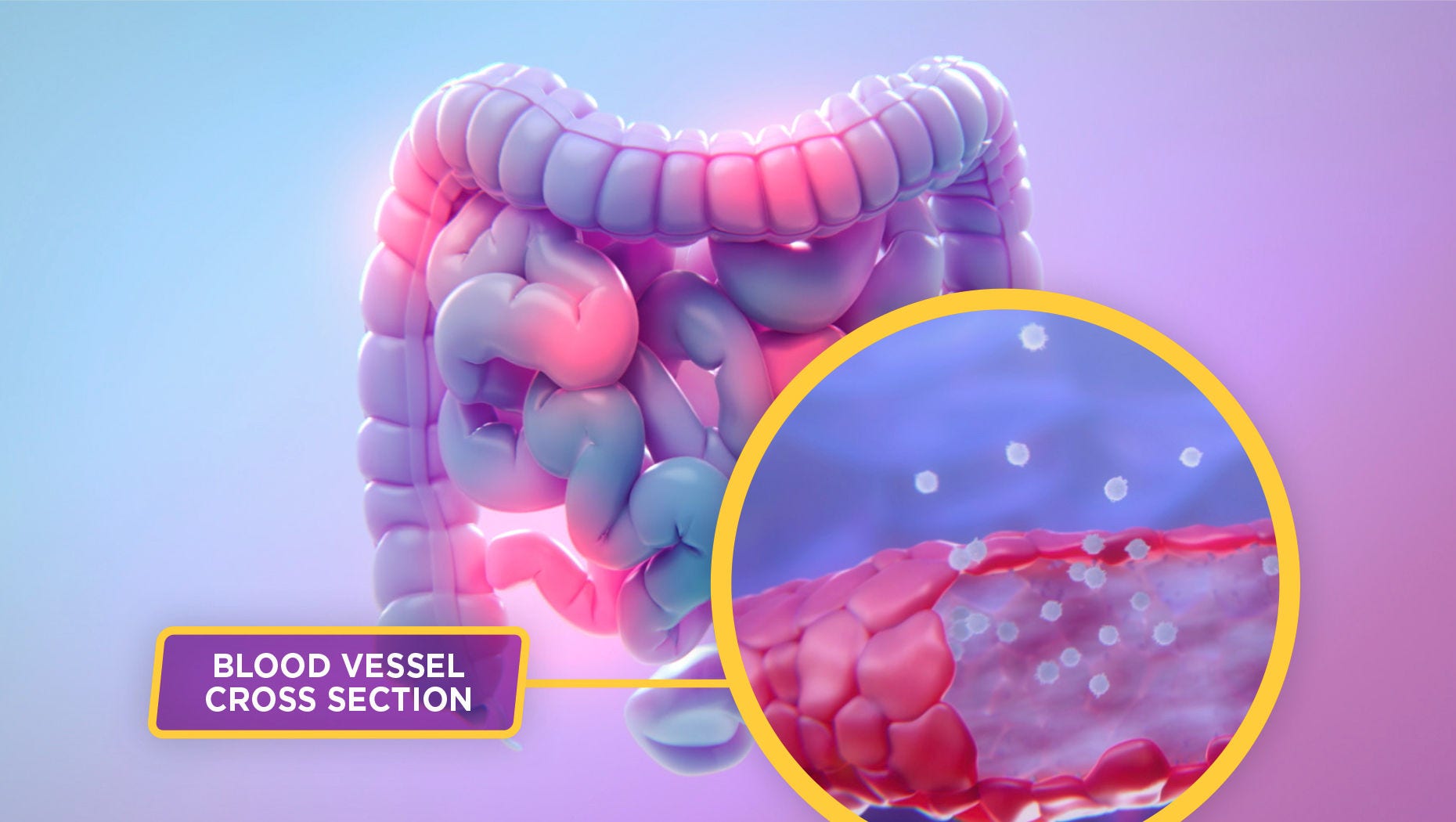
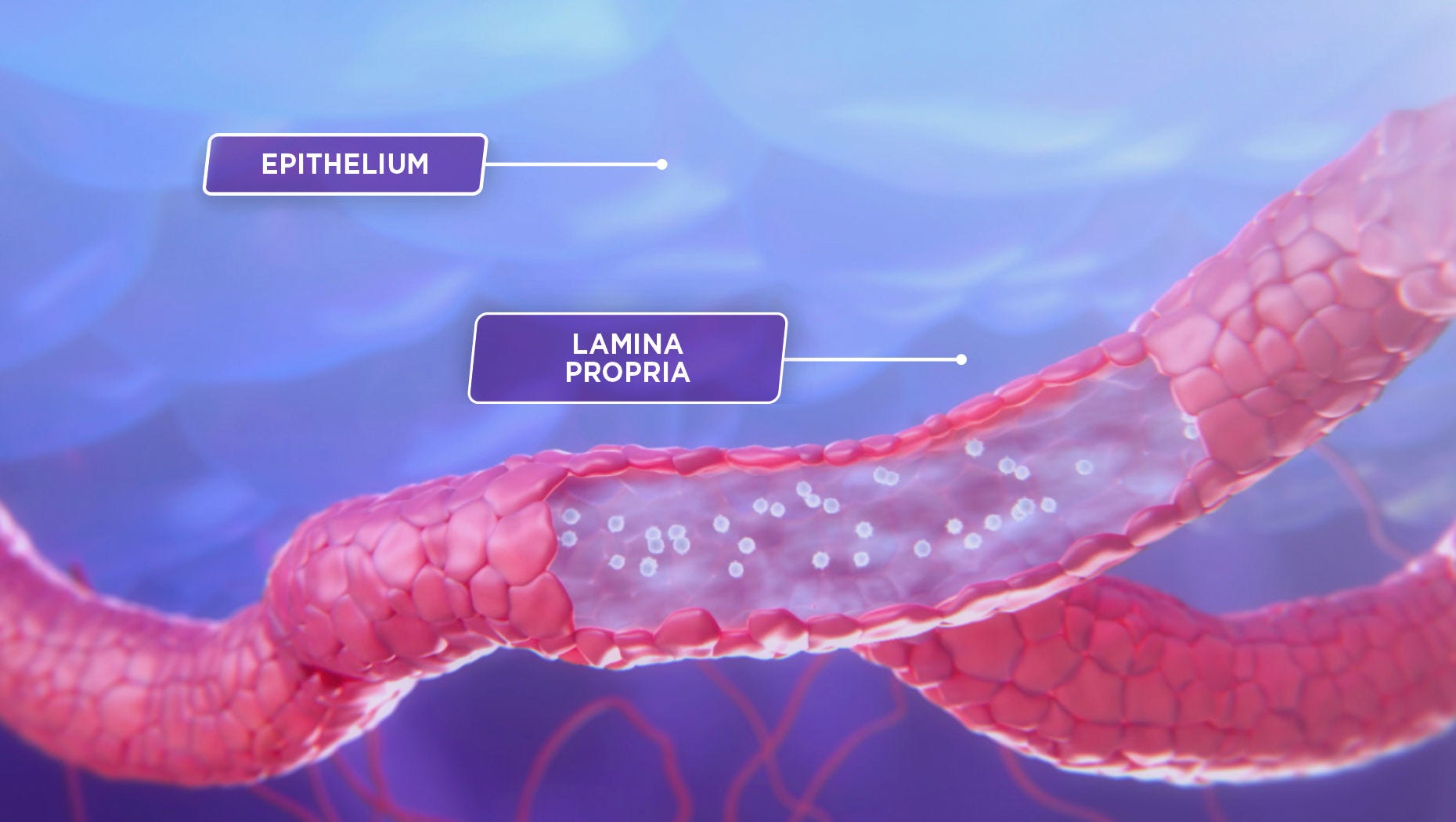
To understand how ENTYVIO works, let’s take a closer look at inflammation in the gastrointestinal tract—the hallmark of ulcerative colitis and Crohn’s disease.1
This inflammation is caused in a large part by an excess of lymphocytes migrating to the gastrointestinal (GI) tract.2
The subset of lymphocytes that preferentially migrate to the GI tract is known to express the α4β7 integrin, which binds to the mucosal addressin cell adhesion molecule-1, or MAdCAM-1, which is primarily expressed on gut endothelial cells.1,3
This interaction is a crucial part of a complex process that allows lymphocytes to exit the bloodstream and enter the body’s tissues.3,4
That’s where ENTYVIO comes in.
It’s a monoclonal antibody that was made to specifically bind to α4β7 and block its interaction with MAdCAM-1.3
As a result, certain lymphocytes are blocked from entering the GI tract and gut inflammation is selectively reduced in ulcerative colitis and Crohn's disease.1,2
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
ENTYVIO is contraindicated in patients who have had a known serious or severe hypersensitivity reaction to ENTYVIO or any of its excipients.
WARNINGS AND PRECAUTIONS
- Infusion-Related and Hypersensitivity Reactions: Infusion-related reactions and hypersensitivity reactions including anaphylaxis, dyspnea, bronchospasm, urticaria, flushing, rash, and increased blood pressure and heart rate have been reported. These reactions may occur with the first or subsequent infusions and may vary in their time of onset from during infusion or up to several hours post-infusion. If anaphylaxis or other serious infusion-related or hypersensitivity reactions occur, discontinue administration of ENTYVIO immediately and initiate appropriate treatment.
- Infections: Patients treated with ENTYVIO are at increased risk for developing infections. Serious infections have been reported in patients treated with ENTYVIO, including anal abscess, sepsis (some fatal), tuberculosis, salmonella sepsis, Listeria meningitis, giardiasis, and cytomegaloviral colitis. ENTYVIO is not recommended in patients with active, severe infections until the infections are controlled. Consider withholding ENTYVIO in patients who develop a severe infection while on treatment with ENTYVIO. Exercise caution in patients with a history of recurring severe infections. Consider screening for tuberculosis (TB) according to the local practice.
- Progressive Multifocal Leukoencephalopathy (PML): PML, a rare and often fatal opportunistic infection of the central nervous system (CNS), has been reported with systemic immunosuppressants, including another integrin receptor antagonist. PML typically only occurs in patients who are immunocompromised. One case of PML in an ENTYVIO-treated patient with multiple contributory factors has been reported. Although unlikely, a risk of PML cannot be ruled out. Monitor patients for any new or worsening neurological signs or symptoms that may include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes. If PML is suspected, withhold dosing with ENTYVIO and refer to neurologist; if confirmed, discontinue ENTYVIO dosing permanently.
- Liver Injury: There have been reports of elevations of transaminase and/or bilirubin in patients receiving ENTYVIO. ENTYVIO should be discontinued in patients with jaundice or other evidence of significant liver injury.
- Live and Oral Vaccines: Prior to initiating treatment with ENTYVIO, all patients should be brought up to date with all immunizations according to current immunization guidelines. Patients receiving ENTYVIO may receive non-live vaccines and may receive live vaccines if the benefits outweigh the risks.
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥3% and ≥1% higher than placebo) were: nasopharyngitis, headache, arthralgia, nausea, pyrexia, upper respiratory tract infection, fatigue, cough, bronchitis, influenza, back pain, rash, pruritus, sinusitis, oropharyngeal pain, pain in extremities, and injection site reactions with subcutaneous administration.
DRUG INTERACTIONS
Because of the potential for increased risk of PML and other infections, avoid the concomitant use of ENTYVIO with natalizumab products and with TNF blockers. Upon initiation or discontinuation of ENTYVIO in patients treated with CYP450 substrates, monitor drug concentrations or other therapeutic parameters, and adjust the dosage of the CYP substrate as needed.
INDICATIONS
Adult Ulcerative Colitis (UC):
ENTYVIO is indicated in adults for the treatment of moderately to severely active UC.
Adult Crohn's Disease (CD):
ENTYVIO is indicated in adults for the treatment of moderately to severely active CD.
DOSAGE FORMS & STRENGTHS
- ENTYVIO Intravenous (IV) Infusion: 300 mg vedolizumab
- ENTYVIO Subcutaneous (SC) Injection: 108 mg vedolizumab
Please see Full Prescribing Information using the link below.
Walk through the ENTYVIO mechanism of action (MOA)
Inflammation
Inflammation in ulcerative colitis and Crohn’s disease is caused in large part by an excess of lymphocytes migrating to the gastrointestinal tract.2
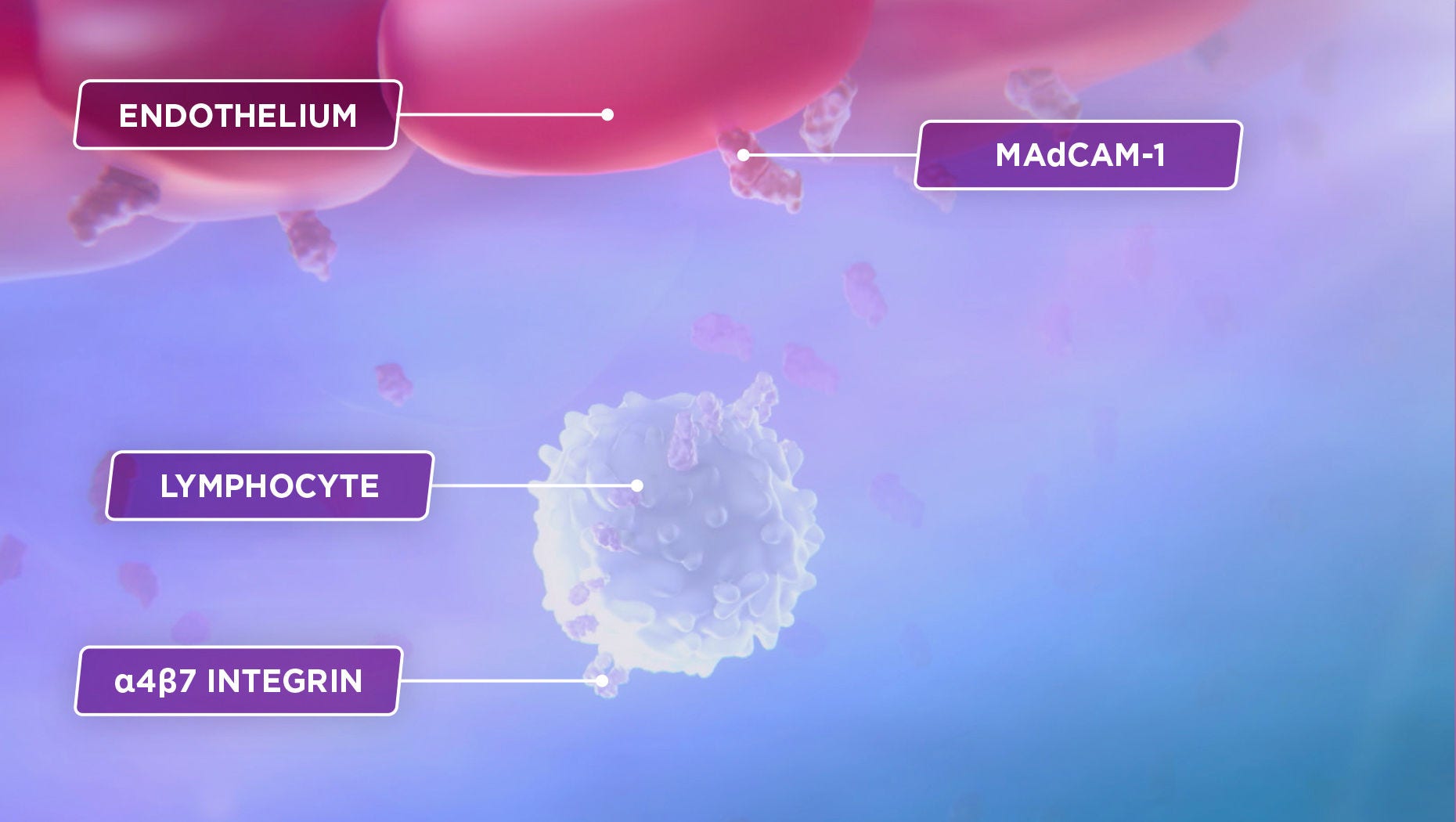
α4β7 and MAdCAM-1
The subset of lymphocytes that preferentially migrate to the GI tract is known to express the α4β7 integrin, which binds to MAdCAM-1, which is primarily expressed on gut endothelial cells.1,3
This interaction is a crucial part of a complex process that allows lymphocytes to exit the bloodstream and enter the body’s tissues.1,4
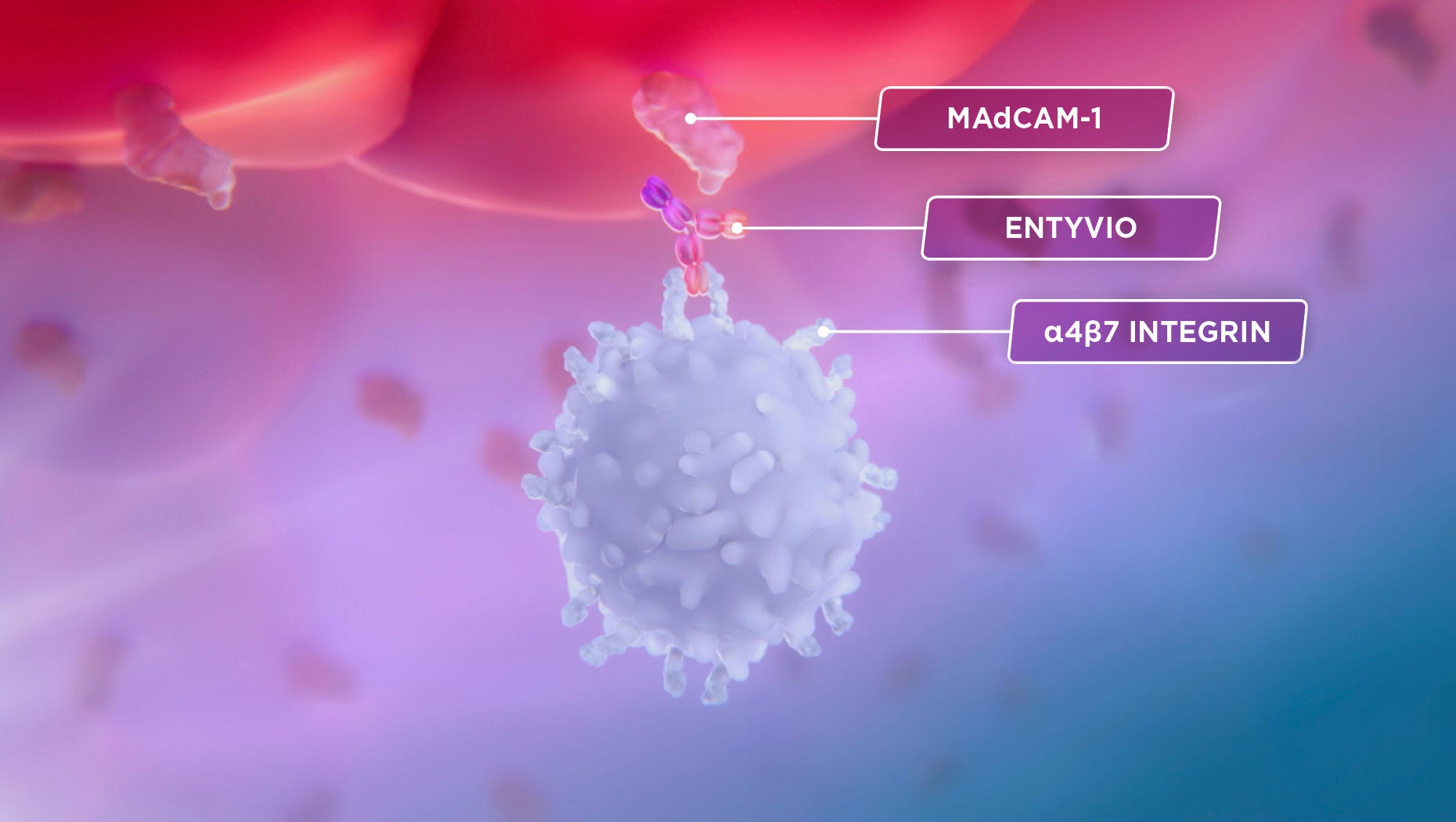
Bind
ENTYVIO is a monoclonal antibody that was made to specifically bind to α4β7 and block its interaction with MAdCAM-1.1
Block
As a result, certain lymphocytes are blocked from entering the GI tract.2,3
Reduce
ENTYVIO selectively reduces gut inflammation in ulcerative colitis and Crohn's disease.1-3
Explore more topics
Ready to start your patient on
ENTYVIO?
Looking for patient support?
The content on this page has been written and
reviewed by Takeda.
IMPORTANT SAFETY INFORMATION
Contraindications
WARNINGS AND PRECAUTIONS
- Infusion-Related and Hypersensitivity Reactions: Infusion-related reactions and hypersensitivity reactions including anaphylaxis, dyspnea, bronchospasm, urticaria, flushing, rash, and increased blood pressure and heart
IMPORTANT SAFETY INFORMATION
Contraindications
ENTYVIO is contraindicated in patients who have had a known serious or severe hypersensitivity reaction to ENTYVIO or any of its excipients.
Warnings and precautions
- Infusion-Related and Hypersensitivity Reactions: Infusion-related reactions and hypersensitivity reactions including anaphylaxis, dyspnea, bronchospasm, urticaria, flushing, rash, and increased blood pressure and heart rate have been reported. These reactions may occur with the first or subsequent infusions and may vary in their time of onset from during infusion or up to several hours post-infusion. If anaphylaxis or other serious infusion-related or hypersensitivity reactions occur, discontinue administration of ENTYVIO immediately and initiate appropriate treatment.
- Infections: Patients treated with ENTYVIO are at increased risk for developing infections. Serious infections have been reported in patients treated with ENTYVIO, including anal abscess, sepsis (some fatal), tuberculosis, salmonella sepsis, Listeria meningitis, giardiasis, and cytomegaloviral colitis. ENTYVIO is not recommended in patients with active, severe infections until the infections are controlled. Consider withholding ENTYVIO in patients who develop a severe infection while on treatment with ENTYVIO. Exercise caution in patients with a history of recurring severe infections. Consider screening for tuberculosis (TB) according to the local practice.
- Progressive Multifocal Leukoencephalopathy (PML): PML, a rare and often fatal opportunistic infection of the central nervous system (CNS), has been reported with systemic immunosuppressants, including another integrin receptor antagonist. PML typically only occurs in patients who are immunocompromised. One case of PML in an ENTYVIO-treated patient with multiple contributory factors has been reported. Although unlikely, a risk of PML cannot be ruled out. Monitor patients for any new or worsening neurological signs or symptoms that may include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes. If PML is suspected, withhold dosing with ENTYVIO and refer to neurologist; if confirmed, discontinue ENTYVIO dosing permanently.
- Liver Injury: There have been reports of elevations of transaminase and/or bilirubin in patients receiving ENTYVIO. ENTYVIO should be discontinued in patients with jaundice or other evidence of significant liver injury.
- Live and Oral Vaccines: Prior to initiating treatment with ENTYVIO, all patients should be brought up to date with all immunizations according to current immunization guidelines. Patients receiving ENTYVIO may receive non-live vaccines and may receive live vaccines if the benefits outweigh the risks.
Adverse reactions
The most common adverse reactions (incidence ≥3% and ≥1% higher than placebo) were: nasopharyngitis, headache, arthralgia, nausea, pyrexia, upper respiratory tract infection, fatigue, cough, bronchitis, influenza, back pain, rash, pruritus, sinusitis, oropharyngeal pain, pain in extremities, and injection site reactions with subcutaneous administration.
Drug interactions
Because of the potential for increased risk of PML and other infections, avoid the concomitant use of ENTYVIO with natalizumab products and with TNF blockers. Upon initiation or discontinuation of ENTYVIO in patients treated with CYP450 substrates, monitor drug concentrations or other therapeutic parameters, and adjust the dosage of the CYP substrate as needed.
INDICATIONS
Adult Ulcerative Colitis (UC):
ENTYVIO is indicated in adults for the treatment of moderately to severely active UC.
Adult Crohn’s Disease (CD):
ENTYVIO is indicated in adults for the treatment of moderately to severely active CD.
Dosage forms & strengths:
- ENTYVIO Intravenous (IV) Infusion: 300 mg vedolizumab
- ENTYVIO Subcutaneous (SC) Injection: 108 mg vedolizumab
Please click for Full Prescribing Information.
References:
- ENTYVIO (vedolizumab) prescribing information. Takeda Pharmaceuticals.
- Soler D, Chapman T, Yang LL, et al. The binding specificity and selective antagonism of vedolizumab, an anti-α4β7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330(3):864-875.
- Wyant T, Fedyk E, Abhyankar B. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J Crohns Colitis. 2016;10(12):1437-1444.
- Fedyk ER, Wyant T, Yang LL, et al. Exclusive antagonism of the α4β7 integrin by vedolizumab confirms the gut-selectivity of this pathway in primates. Inflamm Bowel Dis. 2012;18(11):2107-2119.
- Briskin M, Winsor-Hines D, Shyjan A, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151(1):97-110.
- Wyant T, Leach T, Sankoh S, et al. Vedolizumab affects antibody responses to immunisation selectively in the gastrointestinal tract: randomised controlled trial results. Gut. 2015;64(1):77-83.
- Milch C, Wyant T, Xu J, et al. Vedolizumab, a monoclonal antibody to the gut homing α4β7 integrin, does not affect cerebrospinal fluid T-lymphocyte immunophenotype. J Neuroimmunol. 2013;264:123-126.